
Your introduction
to clinical trials
A clinical trial is a research study where volunteers help test investigational products. These can include drugs, vaccines, devices, or other medical approaches. Investigational products have not yet been approved for the condition being studied.
Clinical trials are carefully designed to find out:
- If the investigational product works
- If it's safe
- If it causes any side effects
Clinical trials are an important way to help improve care for people with different health conditions.
Your introduction
to clinical trials
A clinical trial is a research study where volunteers help test investigational products. These can include drugs, vaccines, devices, or other medical approaches. Investigational products have not yet been approved for the condition being studied.
Clinical trials are carefully designed to find out:
- If the investigational product works
- If it's safe
- If it causes any side effects
Clinical trials are an important way to help improve care for people with different health conditions.

Types of clinical trials
There are different types of clinical trials:
Interventional trials
Doctors give you an investigational product to see if it works and is safe.
Observational trials
Doctors track your health over time without giving you any investigational products. This shows how a condition affects people naturally.
Post-marketing trials
After an investigational product is approved, researchers study it in more people to learn about its long-term safety and how well it works.
All these trials help improve medicine.
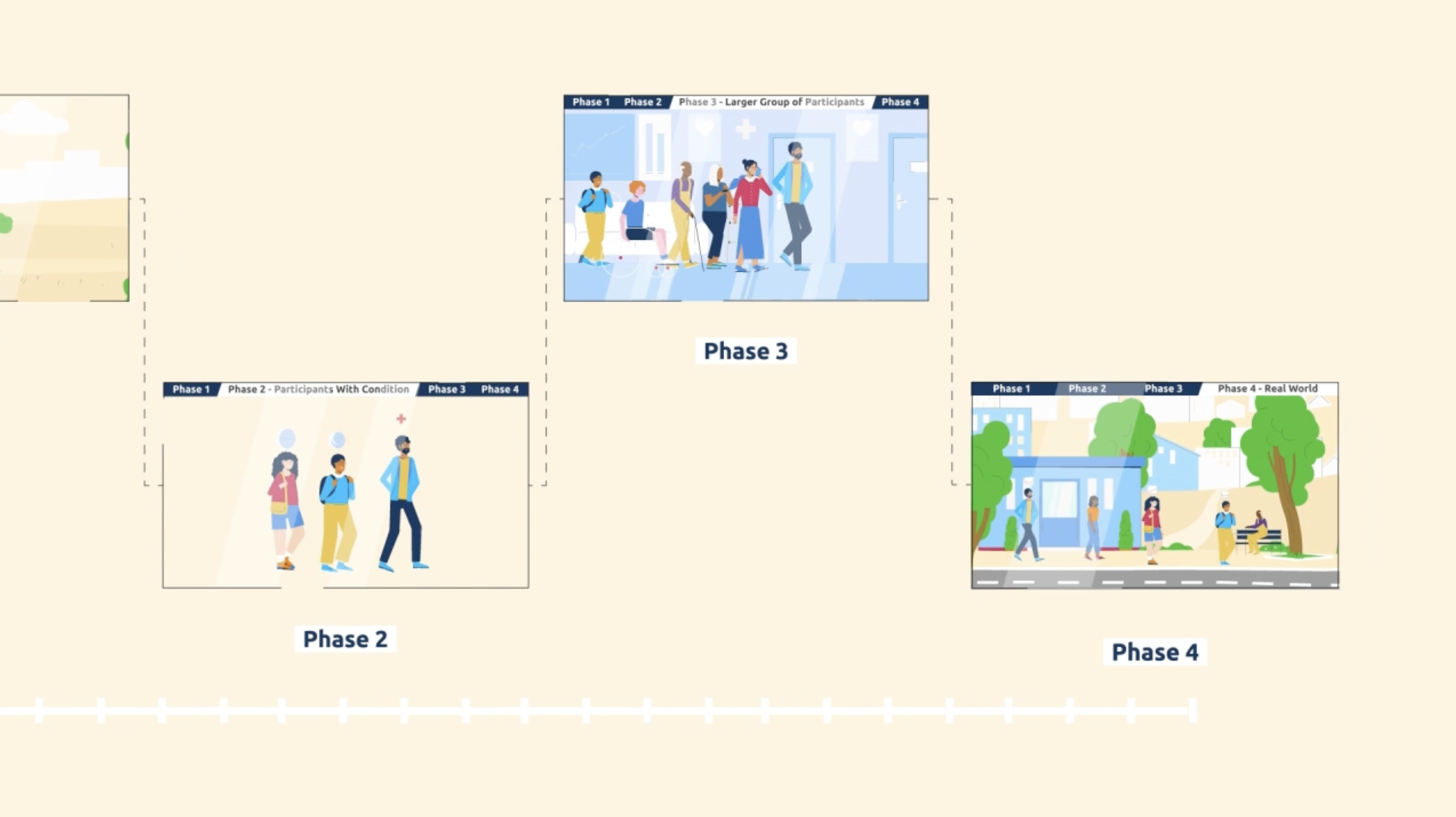
The phases of clinical trials
Clinical research for investigational products follows a clear path with 4 phases. Each phase builds on the one before, testing both safety and how well the product works.
- The safety of the investigational product.
- How the investigational product is processed and removed from the body.
- How the body reacts to different doses.
- Possible side effects.

volunteers
- The safety of the investigational product.
- How the investigational product is processed and removed from the body.
- How the body reacts to different doses.
- Possible side effects.

volunteers
- The safety of the investigational product.
- Whether the investigational product works for the condition being studied.
- What the best dose of the investigational product is.
- The investigational product shows signs it may help.
- The dose is safe enough to use in larger groups.
- The investigational product is still early in development. The goal is to see if it works, but benefits are not guaranteed.
- In some trials, you may get a placebo. A placebo looks like the real investigational product but doesn't have any active medicine in it.
- The safety of the investigational product.
- Whether the investigational product works for the condition being studied.
- What the best dose of the investigational product is.
- The investigational product shows signs it may help.
- The dose is safe enough to use in larger groups.
- The investigational product is still early in development. The goal is to see if it works, but benefits are not guaranteed.
- In some trials, you may get a placebo. A placebo looks like the real investigational product but doesn't have any active medicine in it.
- Safety in bigger groups of people.
- Whether the investigational product works for the condition being studied.
- How the investigational product compares to existing treatment options.
- The investigational product works as well as (or better than) current treatments.
- The dose is still safe when used in many people.
- The investigational product is approved by authorities for public use.
- Depending on the trial, you may get either a placebo or the usual treatment that doctors give for the condition, also called the standard of care.
- These trials often last longer and need more clinic visits.
- Safety in bigger groups of people.
- Whether the investigational product works for the condition being studied.
- How the investigational product compares to existing treatment options.
- The investigational product works as well as (or better than) current treatments.
- The dose is still safe when used in many people.
- The investigational product is approved by authorities for public use.
- Depending on the trial, you may get either a placebo or the usual treatment that doctors give for the condition, also called the standard of care.
- These trials often last longer and need more clinic visits.
Drug trials usually follow a set path with Phases 1 through 4. Some trials may combine phases to study multiple aspects of an investigational product at once.
Trials for medical devices are often called “pre-market” or “post-market” trials. The path for testing new medical devices depends on the type of device as well as other factors.
clinical research

