
How can I join
a clinical trial?
If you're interested in taking part in a clinical trial, there are a few important steps to take before enrolling.
How can I join
a clinical trial?
If you're interested in taking part in a clinical trial, there are a few important steps to take before enrolling.

Step-by-step: what to expect
Start by discussing your interest in clinical trials. Your doctor can help you understand whether a trial might be a good fit for your condition and health goals.
Start by discussing your interest in clinical trials. Your doctor can help you understand whether a trial might be a good fit for your condition and health goals.
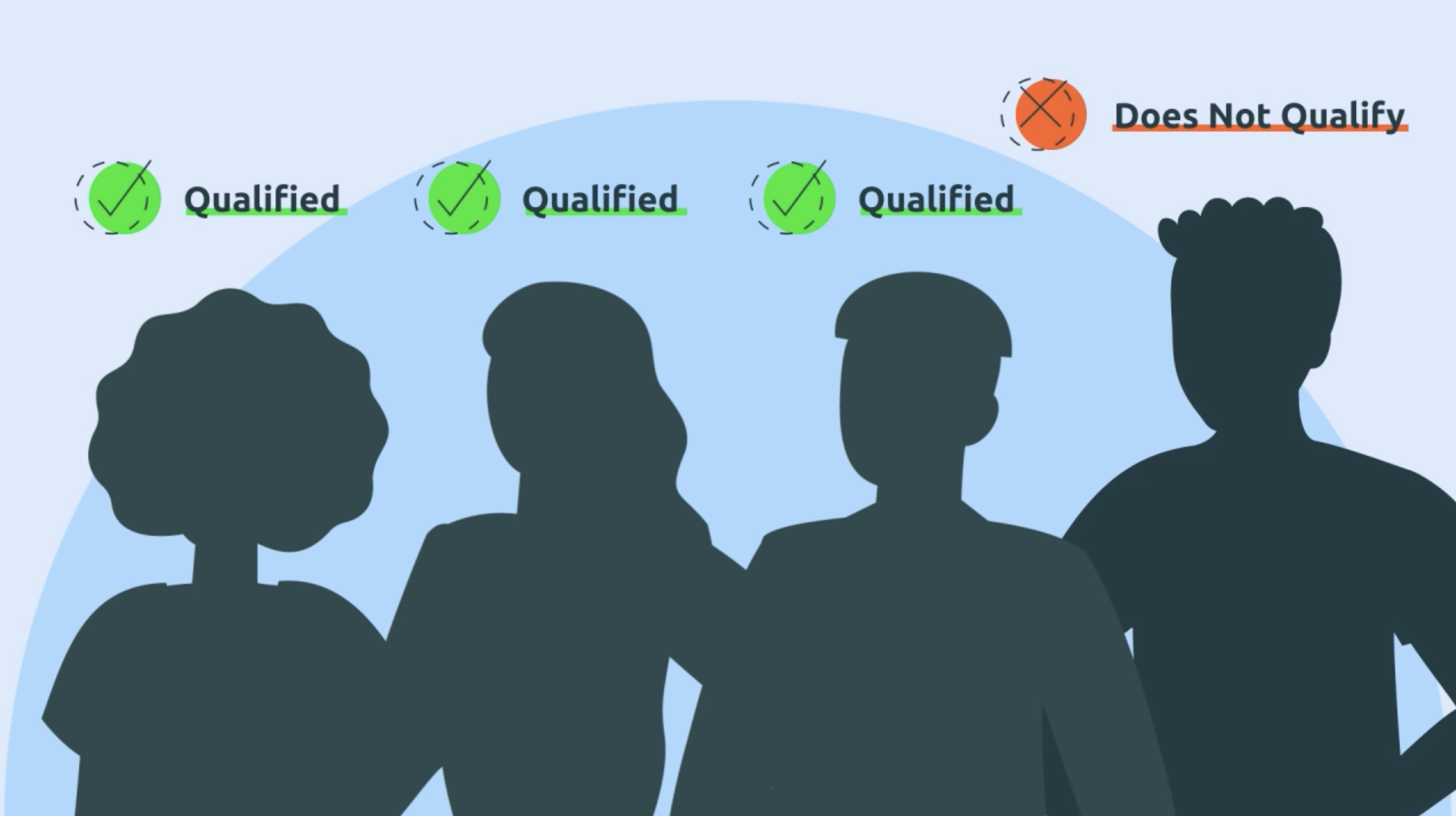
Each clinical trial has specific criteria. When enrolling, you will be asked questions about your:
- Age
- Medical history
- Current medications
- Overall health
This helps determine if you're eligible for the trial.
Each clinical trial has specific criteria. When enrolling, you will be asked questions about your:
- Age
- Medical history
- Current medications
- Overall health
This helps determine if you're eligible for the trial.
If you qualify, the trial team will share detailed information so you can make an informed decision. This includes:
- The purpose of the trial
- What investigational product or procedure is being tested
- Possible benefits and risks
- How long the trial lasts
- What visits and tests are involved
You'll have the chance to ask questions and take time to decide.
If you qualify, the trial team will share detailed information so you can make an informed decision. This includes:
- The purpose of the trial
- What investigational product or procedure is being tested
- Possible benefits and risks
- How long the trial lasts
- What visits and tests are involved
You'll have the chance to ask questions and take time to decide.
If you decide to join, you will be asked to sign an Informed Consent Form. This document confirms that you understand what the trial involves and agree to take part.
Signing the form is voluntary and your consent can be withdrawn at any time.
If you decide to join, you will be asked to sign an Informed Consent Form. This document confirms that you understand what the trial involves and agree to take part.
Signing the form is voluntary and your consent can be withdrawn at any time.
After giving consent, you'll undergo screening tests like:
- Physical exams
- Lab tests (such as blood and urine tests)
- Vital sign checks (like blood pressure and heart rate)
- Questionnaires or surveys
- Other health assessments
These tests help ensure the trial is safe for you and that you're a good match for the trial.
After giving consent, you'll undergo screening tests like:
- Physical exams
- Lab tests (such as blood and urine tests)
- Vital sign checks (like blood pressure and heart rate)
- Questionnaires or surveys
- Other health assessments
These tests help ensure the trial is safe for you and that you're a good match for the trial.
1 min video
Why can't
everyone
participate?
everyone
participate?
Download transcript
Loading...

Can I leave a trial
if I change my mind?
if I change my mind?
Yes. You can stop being in a clinical trial at any time and for any reason. Your participation is completely voluntary.
Whether it's due to changes in your health, schedule, or personal preference - your choice will be respected, and your regular medical care won't be affected.

3 min video
The patient experience
Download transcript
Loading...
Ready to take the next step?
Use the search below to find a trial.
This site is governed solely by applicable U.S. laws and governmental regulations. Please see our Privacy Policy. Use of this site constitutes your consent to application of such laws and regulations and to our Privacy Policy. Your use of the information on this site is subject to the terms of our Legal Notice.
© 2025 Johnson & Johnson Services, Inc.


